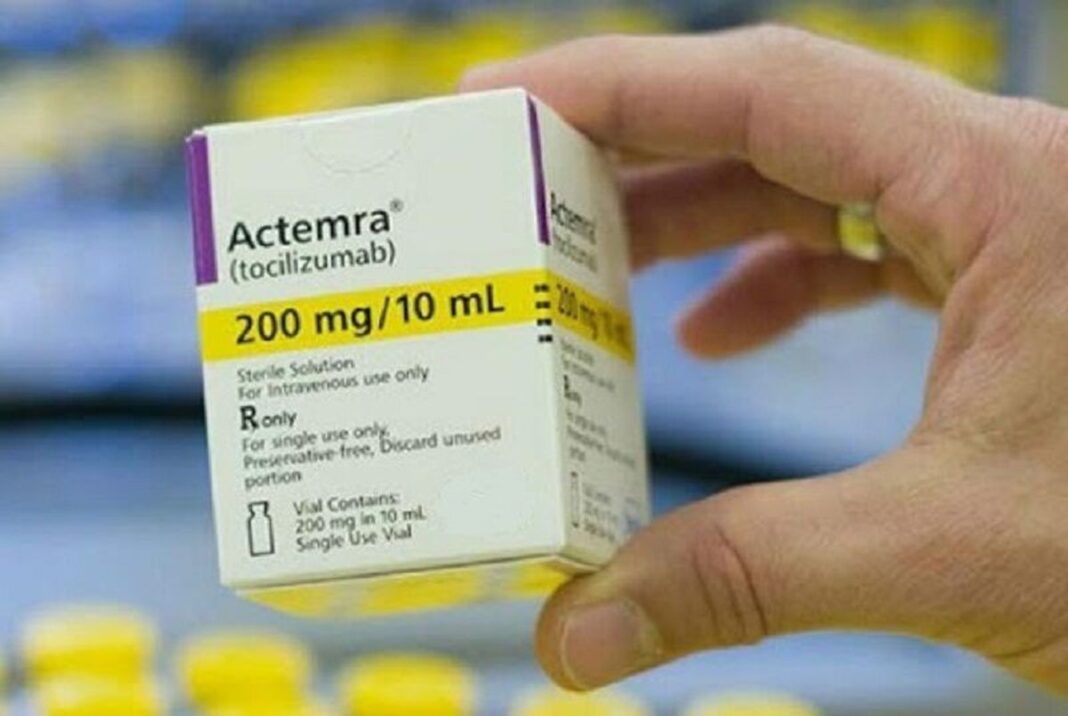
Manager of the Infectious Diseases Ward at Masih Daneshvari Hospital of Tehran says the results of a study conducted by the Association of International Auditors (Empacta) demonstrates that Actemra medicine reduces the risk of COVID-19 pneumonia patients advancing to mechanical ventilation or death by 44 percent.
Dr. Payam Tabarsi said the initial results of the Empacta trial, involving 389 patients and conducted in the US, Brazil, Kenya and a number of other countries, indicate that that Actemra drug -known as tocilizumab made by Swiss company Roche- reduced the risk of COVID-19 pneumonia patients advancing to mechanical ventilation or death by 44%.
Fortunately, the Iranian Actemra, sold under the trademark TEMZIVA, has been approved by the Food and Drug Administration of Iran and is now available in the pharmacies of a number of hospitals, he added.
The high-quality homegrown drug is 38 times cheaper than the Actemra produced by Switzerland’s Roche, Tabarsi said.
Iranian companies have produced homegrown versions of several drugs for the treatment of coronavirus patients after the outbreak of the pandemic in February, including Favipiravir and Remdesivir.
Vice President for Science and Technology Sorena Sattari announced in July that almost all domestic needs for equipment needed to combat COVID-19 have been met by knowledge-based companies.