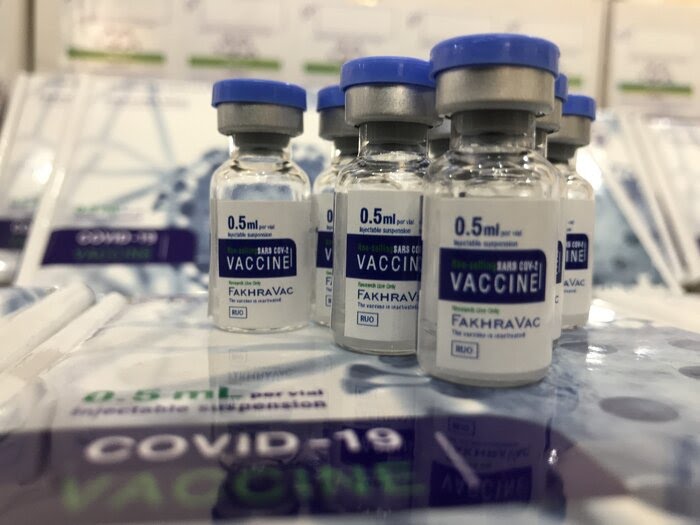
Defense Minister Brigadier General Mohammadreza Ashtiani made the comment while inspecting the process of the construction of a factory that would produce the Fakhra vaccine in Tehran.
He underlined phases one and two of the clinical trial of the vaccine have successfully been conducted.
“After receiving the necessary permits from the Ministry of Health and Medical Education, phase three of the clinical trial of the Fakhra vaccine began, and the vaccine has received the necessary permit for emergency and public use and will be offered for public vaccination in the coming days,” he explained.
“In the meantime, the construction and preparation of the Fakhra Vaccine Production Factory done by experts in the defense industry are in their final stage, and we hope the factory will become fully operational in the near future for the mass-production of vaccines,” he added.
The top general institutes affiliated with the defense ministry have a capacity of producing some 1 million doses of the Fakhra vaccine on a monthly basis.
“When the necessary infrastructure is finalized in December, we can multiply production,” the defense chief noted.